
Hydrogen storage eased
By
Eric Smalley,
Technology Research NewsOne of the biggest challenges to using hydrogen as a fuel is finding a way to store it. The lighter-than-air gas makes the perfect fuel -- it contains three times the energy of liquid hydrocarbons and when it reacts with oxygen to produce energy the only byproduct is water -- but it isn't easy to contain.
Today's hydrogen storage materials hold 2 to 4 percent of their weight in hydrogen, short of the 6.5 percent Department of Energy goal for using hydrogen as automobile fuel.
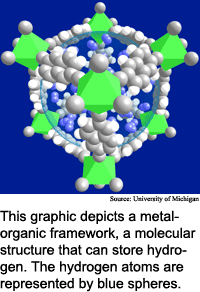
Researchers from the University of Michigan, the University of California at Santa Barbara, the University of South Florida and Arizona State University have discovered a new class of materials, dubbed metal-organic frameworks, that are relatively inexpensive to make and have the potential to reach the 6.5 percent mark. "We are in sight of the DOE goal," said Omar Yaghi, a chemistry professor at the University of Michigan.
The discovery promises to remove the principal stumbling block to hydrogen-powered cars, and the method could be ready for production use within five years.
Hydrogen storage materials act like sponges, capable of filling up with certain gases and later releasing them. The challenge is developing materials that hold useful amounts of hydrogen, and that store and release the hydrogen easily.
Current hydrogen storage systems chemically bind powdered metal hydrides to hydrogen at high temperatures. In November, researchers in Singapore developed a metal material that holds more than 11 percent of its weight in hydrogen, but requires high temperatures and pressures. Researchers are also exploring carbon-based approaches, including carbon nanotubes, but these require very low temperatures.
It is easy to store and retrieve hydrogen using metal-organic frameworks materials, said Yaghi. "Hydrogen can be inserted into the material and then removed reversibly with no change to the storage medium," he said. When the materials are exposed to hydrogen at room temperature and under modest pressure, they take it up immediately, he said.
This is possible because hydrogen is adsorbed by rather than chemically bound to the storage material, said Yaghi. Adsorption is the process of gas or vapor atoms sticking to a surface. "The hydrogen is physically attracted to the walls of the [material's] pores," he said. "This attraction makes it possible to stuff more hydrogen molecules into a small area without requiring either low temperatures or high pressures."
Metal-organic frameworks are exceptionally porous at the molecular scale, with surface areas of more than 3,000 square meters per gram, according to Yaghi. They are "basically scaffolds of linked rods," he said.
The materials have several other advantages, said Yaghi. They're made from low-cost starting materials including zinc oxide, which is used in sunscreen lotion, and terephthalate, which is a component of plastic soda bottles. They are simple to make, and manufacturing yields are high, he said.
The researchers previously showed that metal-organic frameworks can absorb voluminous quantities of nitrogen and organic vapors, said Yaghi. "Given the importance of hydrogen as a fuel, we sought to examine the hydrogen storage capabilities," he said.
The researchers' showed that it is possible to design metal-organic frameworks materials that absorb incrementally more hydrogen. Their best prototypes store two percent of their weight in hydrogen, but the materials have the potential to store much more, said Yaghi. "We have shown that we can systematically increase the hydrogen storage capacity of these materials, thus identifying a clear path toward achieving the DOE hydrogen storage goal," he said
The researchers are currently working on increasing the hydrogen capacity of the materials and also on better understanding the reasons the materials are able to absorb so much hydrogen, said Yaghi.
The researchers are also collaborating with BASF Corporation to use the materials in practical applications. It will take from two to five years of development before the material can be used in practical applications, Yaghi said.
Yaghi's research colleagues were Nathaniel Rosi, David T. Vodak and Jaheon Kim from the University of Michigan, Jurgen Eckart from the University of California at Santa Barbara and the Los Alamos National Laboratory, Muhamed Eddaoudi from the University of South Florida, and Michael O'Keefe from Arizona State University. The work appeared in the May 16, 2003 issue of Science. The research was funded by the National Science Foundation (NSF), the Department of Energy (DOE) and BASF Corporation.
Timeline: 2-5 years
Funding: Corporate, Government
TRN Categories: Energy; Materials Science and Engineering
Story Type: News
Related Elements: Technical paper, "Hydrogen Storage in Microporous Metal-Organic Frameworks," Science, May 16, 2003.
Advertisements:
May 21/28, 2003
Page One
Hydrogen storage eased
Flexible display slims down
Simulated evolution gets complex
Model explains market movements
News briefs:
Big qubits linked over distance
Software maps group work
Magnesium batteries show mettle
Nanotubes smash length record
DNA sensor changes color
Sensor serves up body slices
News:
Research News Roundup
Research Watch blog
Features:
View from the High Ground Q&A
How It Works
RSS Feeds:
News
Ad links:
Buy an ad link
| Advertisements:
|
 |
Ad links: Clear History
Buy an ad link
|
TRN
Newswire and Headline Feeds for Web sites
|
© Copyright Technology Research News, LLC 2000-2006. All rights reserved.