
How
metallic are metal nanotubes?
By
Kimberly Patch,
Technology Research NewsAs researchers examine carbon nanotubes more closely, they're finding that the tiny rolls of graphite are more complicated than they may have first appeared.
Researchers from Harvard have examined metallic nanotubes and found that a nanotube's environment affects whether it is truly metallic or not. The finding throws at least a small complication into plans to eventually use the nanotubes as ready-made wires for nanoscale electronics.
Nanotubes come in two basic types: metal, which allow electrical current to flow freely, and semiconducting, which contain energy gaps that slow current. Electrons and their charge opposites, positive holes, inhabit certain orbits around an atom; bandgaps are the spaces between orbits where charges cannot flow.
Nanotubes can be smaller than one nanometer in diameter and are made of one-molecule-thick sheets of graphite that roll up to form the tubes. A combination of the angle of the roll and the diameter of the tube determines its electrical properties.
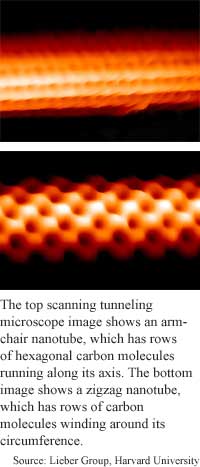
There are two basic types of metal nanotubes: armchair and zigzag. The difference is in the direction the graphite sheet is rolled. The sheet's hexagonal rows of carbon molecules run along the nanotube axis in armchair nanotubes and around it's circumference in Zigzag nanotubes.
The researchers found that zigzag nanotubes contain energy gaps that stop current from flowing freely. "They're truly semiconductors," said Charles Lieber, a chemistry professor at Harvard University.
The way armchair nanotubes conduct is a little more complicated. When bundled with other nanotubes, they contain pseudo energy gaps that lone armchair nanotubes do not. "Thus... to obtain good electrical interconnects, [or] wires, we will need to use armchair carbon nanotubes, with the tubes isolated -- not in bundles. Only these will be true metals," he said.
The pseudo gaps are actually caused by the molecular interactions among two or more nanotubes, which destroy the rotational symmetry that gives lone armchair nanotubes metallic properties, said Lieber. Rotational symmetry simply means the tube looks the same when rotated a certain amount. For instance a wheel with six spokes will look the same after it is rotated 60 degrees.
The researchers do not know precisely how much the pseudo energy gaps in bundled armchair nanotubes will slow current. "We do not yet know how dramatic an effect the pseudogap will have on the conducting properties. However, all theory would suggest that this type of gap will degrade their conducting properties," said Lieber.
The research shows that controlling the environment around the nanotube is important, said Lieber. "It's not so simple as you just take something that should be a metal and throw it on a surface," he said.
In light of the research, it may be useful to find a way to isolate armchair nanotubes. This is tricky, because nanotubes like to stick together, Lieber said. "You don't get single sheets of graphite floating around -- they have a very strong attraction for one another," he said.
One way to both sort nanotubes into the correct types and keep conducting armchair nanotubes apart is to grow them in place. "You could probably grow isolated tubes, but I just don't know how to at this time," said Lieber. "We know now what needs to be done to get the electronic properties. It's not physically impossible, there's just some hard work that's going to have to be done."
These gaps could also be used in a positive way, Lieber added. Both the true energy gaps and the pseudo gaps make the nanotubes conductivity sensitive, meaning the conductivity changes when chemicals bind to the tubes. Because of this, these types of nanotubes could be used as nanoscale chemical or biological sensors, he said.
The researchers are now more closely examining the armchair nanotubes' pseudo gap, including how it forms and how environment can be used to control this type of gap, said Lieber. They are looking to better understand the role of defects, or misalignments in determining nanotube electronic properties, he said.
It's nice work, said Dan Ralph, an associate professor of physics at Cornell University. "It turns out that the gap is pretty small," however, which means the tubes may still be useful as wires, at least for room temperature applications, Ralph said. "There may be small differences in conductivity costs, but by tens of percent rather than big orders of magnitude," he said.
The gap may loom larger for low temperature applications because colder electrons have less thermal energy. It also may turn out to matter more after larger problems like resistance between a contact and a nanotube, which is often comparable to the resistance of the whole tube, have been solved, he said.
Researchers generally agree that it will be more than five or even 10 years before nanotubes can be used practically in circuitry.
Lieber's research colleagues were Min Ouyang, Jin-Lin Huang and Chin Li Chang of Harvard University. They published the research in the April 27, 2001 issue of the journal Science. The research was funded by the National Science Foundation (NSF).
Timeline: 5-10 years
Funding: Government
TRN Categories: Nanotechnology
Story Type: News
Related Elements: Technical paper, "Energy Gaps in 'Metallic' Single Walled Carbon Nanotubes," Science, April 27, 2001.
Advertisements:
May 30, 2001
Page One
VR tool aims high
Bulk nanotubes make clean crystals
Engine fires up electrical devices
Microscopic stamps make nanotech devices
How metallic are metal nanotubes?
 the
the News:
Research News Roundup
Research Watch blog
Features:
View from the High Ground Q&A
How It Works
RSS Feeds:
News
Ad links:
Buy an ad link
| Advertisements:
|
 |
Ad links: Clear History
Buy an ad link
|
TRN
Newswire and Headline Feeds for Web sites
|
© Copyright Technology Research News, LLC 2000-2006. All rights reserved.