
Chip
juggles droplets
By
Kimberly Patch,
Technology Research NewsWe've become accustomed to using electric pulses to represent the binary bits of digital information, and manipulating these bits by moving them through the logic circuits of computers. Although drops of liquid are very different from digital bits, researchers have found a way to transport water that makes working with minute amounts of fluids a lot like computing.
Researchers from Duke University and Nanolytics are using electricity to push around, carve up and mix tiny droplets of liquid on a chip.
The process could lead to reconfigurable biology and chemistry labs-on-a-chip that perform many different tasks that involve controlling minute amounts of matter.
The electronic transport process, which also automatically mixes the liquid within droplets, replaces the network of channels usually required to contain and direct liquids on chips with a smooth surface dotted with electrodes. The idea is that many of the advantages of the digital approach in microelectronics might apply to a microfluidics system as well, said Michael Pollack, a researcher at Duke University.
Sliding drops around in the absence of channels makes for a physically simple but flexible chip, said Pollack. "Because we actuate the droplets directly, there is no need for pumps or valves in the system," he said. The lack of channels, in turn, makes the entire operation of the chip potentially reconfigurable, he said.
Most other microfluidics devices are continuous-flow systems where liquids are driven through a network of channels permanently etched in glass, plastic or silicon, said Pollack. Those systems work well for specific applications, but "are relatively inflexible and difficult to integrate" to form more complicated systems, he said.
Because each droplet in the Duke/Nanolytics system can be controlled independently, "complicated systems or operations can be built up from discrete parts that operate and interact in well-understood ways," said Pollack. This modular approach is similar to the way software programs are made, and makes complicated systems much simpler to design and control, he said.
Ultimately, the technique could be used for a general-purpose biochemistry workstation, he said. "A researcher or clinician could load sample and reagent droplets into the chip and then select or write a program for carrying out any protocol using these initial droplets as sources for forming hundreds of thousands of smaller droplets," said Pollack.
The key to using electricity to transport water across a surface is surface tension. The researchers sandwiched droplets between glass plates, and used electric fields generated by electrodes lining the bottom plate to change the droplets' surface tension, which, in turn, changed their shapes.
This happens because the combination of a droplet, the insulating surface of a plate, and an electrode makes a kind of capacitor that can store electrostatic energy. As the droplet gains energy, the chemical tension between the droplet and surface is reduced, causing the droplet to spread out.
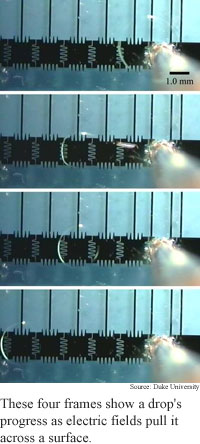
By applying different electric fields to different parts of a droplet, the researchers were able to move, join and split droplets on the surface.
Using a series of electric field changes, the researchers caused droplets to travel as quickly as 20 centimeters per second. "By activating the patterned control electrodes on the bottom plate we can create a surface tension well, and if the droplet is overlapping a portion of one of these wells, it will move," said Pollack.
To produce continuous motion, the researchers repeated the process many times. "As long as the droplet maintains an overlap with the adjacent electrodes, we can transport it through successive alignments to the next... electrode," Pollack said.
A key attribute of the process is that the size of the droplet does not affect its speed, said Pollack.
Because smaller droplets can cover the same distance in the same time as much larger droplets, as the system is scaled down to smaller sizes, the speed of the droplets relative to the size of the system will increase. "For example, at 15 centimeters per second, a 1.5 millimeter diameter droplet can be moved 100 times its length in a second, but a 0.15 millimeter diameter droplet can be moved 1,000 times its length" per second, he said.
This is 100 times faster than previously demonstrated methods of moving droplets using electricity, 40 times faster than electrochemical actuation, and more than 2,000 times faster than light-driven actuation, according to Pollack.
In addition, the researchers' method includes a built-in mixing function because it causes droplets to roll, rather than slide across a surface. "This is very important because it means that there is a circulation of liquid within the droplet as it moves," which quickly and efficiently mixes its contents, said Pollack.
Getting the contents of a very small amount of liquid to mix is an issue. In a channel as small as the width of a human hair, for instance, water doesn't slosh like it does in an eight-ounce glass, but moves more like honey.
The work is an important step forward, said Glenn Walker, a researcher at the University of Wisconsin at Madison. The technique sidesteps a common problem in microfluidics, which is how to move, mix and dispense fluid without harming the sample, said Walker. "The technique... is one answer to this problem," he said.
The work is "beautiful," said Menno Prins, a scientist at Phillips Research in the Netherlands. "The control of formation, splitting and joining of droplets by electrowetting has not been demonstrated before to such [an] extent," he said.
The method should find use in future lab-on-a-chip devices, said Prins. The method "will become very powerful when the researchers are able to extend the technology to wider classes of fluids," he said.
It will be exciting to see the researchers' technique adapted to biological experiments and assays, said Walker. Assays are tests that screen samples for particular microbes or chemicals. The challenge will be avoiding sample contamination and surface fouling, which are common problems in working with biological substances, he added.
The researchers' next step is to demonstrate that the technique is useful for biological assays, said Pollack. "We have shown that all of the individual parts work -- droplet forming, transport, mixing, splitting -- and that we can transport droplets containing biological solutions, so now we are interested in putting these parts together to demonstrate that a range of biological assays can be carried out on the chip," he said.
There are potential applications for the technology "wherever there is a need to do biology or chemistry in a highly automated or high-throughput way," said Pollack. "For example, in clinical diagnostics, environmental monitoring... drug discovery or genotyping applications," he said.
The droplet technology could be ready for practical application in two to four years, Pollack added.
Pollack's research colleagues were Alexander D. Shenderov of Nanolytics in Raleigh, North Carolina and Richard B. Fair of Duke University. They published the research in the second quarter, 2002 issue of the journal Lab on a Chip. The research was funded by the Defense Advanced Research Projects Agency (DARPA).
Timeline: 2-4 years
Funding: Government
TRN Categories: Microfluidics and BioMEMS
Story Type: News
Related Elements: Technical paper, "Electrowetting-Based Actuation of Droplets for Integrated Microfluidics," Lab on a Chip, second quarter, 2002; Related technical paper, "How to Make Water Run Uphill", Manoj Chaudhury and George Whitesides, Science, June 12, 1992.
Advertisements:
September 4/11, 2002
Page One
Chip juggles droplets
Software turns reading into writing
Radio ID locks lost laptops
Quantum software gets the picture
Laser blasts make memory
News:
Research News Roundup
Research Watch blog
Features:
View from the High Ground Q&A
How It Works
RSS Feeds:
News
Ad links:
Buy an ad link
| Advertisements:
|
 |
Ad links: Clear History
Buy an ad link
|
TRN
Newswire and Headline Feeds for Web sites
|
© Copyright Technology Research News, LLC 2000-2006. All rights reserved.