
Chemists
brew tiny wires
By
Eric Smalley,
Technology Research NewsThere are two ways to make the smaller circuits and electronic components that promise to underpin tomorrow's technologies: improve today's top-down approach of using tools to manufacture circuits, and develop a bottom-up approach of having the circuits build themselves molecule by molecule.
Though it may never be possible to produce entire computer chips simply by mixing the right chemicals in the right order, the low cost and small sizes made possible by the bottom-up approach could revolutionize electronics. This potential, along with recent advances by chemists and materials engineers who are coaxing useful structures to self-assemble, is fueling the nanotechnology boom.
A major challenge to making self-assembling electronics is that materials that readily form structures tend to be poor electrical conductors. A research team led by a chemist from the University of Pennsylvania has found a way to coax two types of materials -- one electrically insulating and the other electrically conducting -- to combine into microscopic insulated wires.
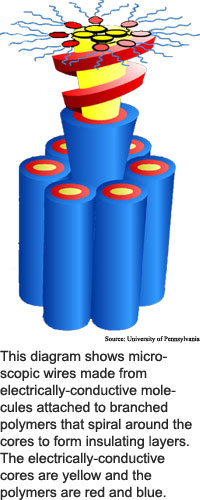
The method produces trillions of nanowires at a time, arranged vertically to form a thin polymer, or plastic, film.
The researchers made the wires by attaching electrically-conductive molecules to the bases of branched polymers. Polymers are long, chain-like molecules that can easily be made to change shape.
The researchers designed their wedge-shaped branched polymers, or dendrimers, to attract each other, and to connect to form spiral cylinders, said Virgil Percec, a professor of chemistry at the University of Pennsylvania.
When the dendrimers come together, they form cylinders around the conductive molecules attached to the points of the dendrimer wedges. The conductive molecules stack up, forming sets of four, five or seven columns encased within the dendrimer spiral.
The dendrimers electrically insulate and keep moisture away from the electrically-conductive columns. The method could be used with many kinds of conductive materials, said Percec. "A large variety of electronically active molecules can be incorporated in the center of the cylinders."
The self-assembled electric wires resemble strands of DNA, with the conductive molecules in place of DNA's base pairs, and the dendrimers in place of DNA's sugar-phosphate backbone, said Percec. The wires are about 10 nanometers in diameter, which is about the width of 100 atoms. The wires are as long as the thickness of the plastic film, which ranges up to 1,000 nanometers. A nanometer is one millionth of a millimeter.
The researchers have caused these self-assembling, self-repairing insulated nanowires to form perpendicular to surfaces and between two surfaces such as a pair of electrodes, said Percec.
The nanowires could be used in photovoltaics cells, which turn light into electricity, and to make smaller transistors than are possible with today's chipmaking processes, Percec said.
The researchers' work is "truly remarkable," said Hicham Fenniri, an assistant professor of chemistry at Purdue University. The process introduces a new level of control over the supramolecular organization of optoelectronic materials, he said. "I foresee numerous applications in molecular electronics and photonics."
The research describes a "clever way to hit what may be a sweet spot" between the higher conductivity of organic crystals and easier-to-work-with polymers, said Vincent Crespi, an associate professor of physics at Pennsylvania State University. "The conductivity isn't quite as good as a single-crystal organic material, and the processing isn't quite as easy as [that of] more disordered polymer material, but [the nanowires have] a combination of conductivity and processability that is unmatched by either," he said.
Practical applications for the supramolecular wires will be possible in less than two years, said Percec.
The researchers' next steps are to improve the conductivity of the wires and to use them in technological applications, said Percec. The first practical application may be in photovoltaics, he said.
Percec's and Singer's research colleagues were Martin Glodde, Tushar-Kanti Bera, Yoshiko Miura, Venkatachalapathy Balagurusamy and Paul Heiney of the University of Pennsylvania, Kenneth David Singer and Irina Shiyanovskaya of Case Western Reserve University, Ingo Schnell and Almut Rapp of the Max Planck Institute, and Steven Hudson and H. Duan of the National Institute of Standards and Technology (NIST).
They published the research in the September 26, 2002 issue of the journal Nature. The research was funded by the National Science Foundation (NSF), the Air Force Office of Scientific Research (AFOSR), the Army Research Office (ARO), the Office of Naval Research (ONR), the German Federal Ministry of Education and Research (BMBF), and the Humboldt Foundation.
Timeline: < 2 years
Funding: Government; Private
TRN Categories: Biological, Chemical, DNA and Molecular Computing; Nanotechnology; Chemistry
Story Type: Is
Related Elements: Technical paper, "Self-organization of Supramolecular Helical Dendrimers Into Complex Electronic Materials," Nature, September 26, 2002
Advertisements:
October 16/23, 2002
Page One
Chemists brew tiny wires
Voiceprints make crypto keys
Stamp corrals tiny bits
Net devices arranged fractally
Quantum scheme lightens load
News:
Research News Roundup
Research Watch blog
Features:
View from the High Ground Q&A
How It Works
RSS Feeds:
News
Ad links:
Buy an ad link
| Advertisements:
|
 |
Ad links: Clear History
Buy an ad link
|
TRN
Newswire and Headline Feeds for Web sites
|
© Copyright Technology Research News, LLC 2000-2006. All rights reserved.