
Fuel cell converts waste to
power
By
Kimberly Patch,
Technology Research NewsFuel cells extract energy from fuel chemically rather than burning it, which in general is more efficient and produces less pollution than combustion engines that burn fuel.
Hydrocarbons like fossil fuels and plant matter are widely used as fuel in fuel cells, but they produce carbon monoxide as a waste product. This carbon monoxide gets in the way of the fuel cell reaction.
Carbon monoxide is usually removed using the water-gas shift reaction, which uses water to convert carbon monoxide to carbon dioxide and hydrogen. The reaction is relatively slow and occurs at temperatures of at least 227 degrees Celsius, requiring fuel cells to contain heating and cooling equipment and a supply of water.
Researchers from the University of Wisconsin at Madison have found a way to use the carbon monoxide to produce more energy in a reaction that can take place at room temperature.
The method could eventually be used in portable systems that use renewable fuel produced from plant matter, said James Dumesic, a professor of chemical and biological engineering at the University of Wisconsin at Madison. The process could also be used to treat wastewater and contaminated gas streams, he said.
Fuel cells are made up of electrodes, an electrolyte -- a pool of chemicals that bathes the electrodes -- and fuel, which is used up as the cell produces energy. Like a battery, fuel cells generate a flow of electricity by pushing electrons out one electrode and receiving them back through a second electrode.
A fuel cell generates electricity when fuel flows into a negative electrode, or cathode, and air flows over the positive electrode, or anode. Oxygen reacts with water in the electrolyte and forms hydroxide ions, and the fuel reacts with the hydroxide ions to form water; this releases two electrons per hydrogen molecule.
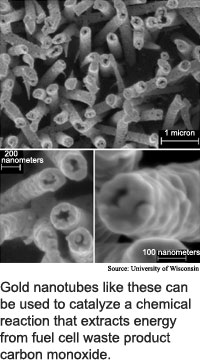
The researchers got the idea for a different kind of fuel cell when they observed that the carbon monoxide left over from this reaction could be combined with oxygen to release more electrons during a water-based reaction using membranes made from gold nanotubes. The tiny tubes are 200 nanometers in diameter, or one-fifth the diameter of an E. coli bacterium.
At the nanoscale in an aqueous solution, gold catalyses chemical reactions fairly quickly even at room temperature, according to Dumesic. With this in mind, the researchers added a chemical reactor step to the fuel cell process. The reactor consists of a gold nanotubes membrane surrounded by water that contains dissolved polyoxometalate, a metal complex that has a high affinity for electrons.
The gold nanotubes convert carbon monoxide and liquid water to carbon dioxide, hydrogen ions and electrons. The polyoxometalates are positively charged and thus readily combine with the negatively-charged electrons.
The polyoxometalates are then pumped past the fuel cell anode along with the hydrogen molecules. There the anode strips off electrons from the polyoxometalates and hydrogen. The polyoxometalates, restored to a positive charge, are returned to the chemical reactor to continue the carbon monoxide processing cycle.
In order to make the system work, the researchers had to make their own fuel cell electrodes. The platinum electrodes normally used in fuel cells tend to have hydrophobic properties in order to avoid problems with water condensation, said Dumesic. Water condensation can inhibit the transport of hydrogen and oxygen.
The researchers realized that the standard type of electrodes did not allow the fuel cells to take advantage of their observation that the catalytic effects of the gold nanotubes in carbon monoxide oxidation are greatly improved in water. "Especially the anode [had to] be strongly hydrophilic to facilitate the aqueous polyoxometalate solution transport," Dumesic said.
The researchers' made one type of hydrophilic electrode from gold nanotubes and another from carbon membranes. "We spent a substantial time to resolve this issue and [are still] improving the performance of this electrode," he said.
The researchers' system has the potential to reduce carbon monoxide levels to below 1,000 parts per million, said Dumesic. "Our process can clean the hydrogen stream by removing carbon monoxide [at a] very high rate even at room temperature, without significantly consuming the hydrogen."
Unlike the water-gas shift reaction, the researchers' process does not require energy to remove carbon monoxide from a stream of hydrogen, said Dumesic.
The researchers experiments show that gold nanoparticles that are 7 to 10 nanometers in diameter would make the chemical reactor step more efficient than the nanotubes in the researchers' current prototype because of the greater surface area of the smaller bits of gold, according to Dumesic.
Using the fuel ethylene glycol, which is produced from corn, the process can extract about 60 percent of the energy that can be produced from octane combustion, Dumesic said.
The researchers are refining the method to develop portable, practical fuel cells that run on fuel produced from biomass, said Dumesic. The method could be ready for practical use in five years, he said.
Dumesic's research colleagues were Won Bae Kim, T. Voitl and J. Rodriguez-Rivera. The work appeared in the August 26, 2004 issue of Science. The research was funded by the National Science Foundation (NSF), DaimlerChrysler Corporation and the Department of Energy (DOE).
Timeline: 5 years
Funding: Corporate, Government
TRN Categories: Energy; Materials Science and Engineering
Story Type: News
Related Elements: Technical paper, "Powering Fuel Cells with CO via Aqueous Polyoxometalates and Gold Catalysts," Science, August 26, 2004
Advertisements:
September 22/29, 2004
Page One
Fuel cell converts waste to power
Bank transfer demos quantum crypto
Agent model yields leadership
Flexible sensors make robot skin
Briefs:
Microscope etches ultrathin lines
Nanowire makes standup transistor
Plastics ease nanotube circuits
Virus forms nano template
Photo molecules flip current
Nanotubes on cloth fire electrons
News:
Research News Roundup
Research Watch blog
Features:
View from the High Ground Q&A
How It Works
RSS Feeds:
News
Ad links:
Buy an ad link
| Advertisements:
|
 |
Ad links: Clear History
Buy an ad link
|
TRN
Newswire and Headline Feeds for Web sites
|
© Copyright Technology Research News, LLC 2000-2006. All rights reserved.